Strength of Acids and Bases
Strength of Acids and Bases: Overview
This Topic covers sub-topics such as pH Scale, Universal Indicator, Importance of pH in Everyday Life, pH of Acidic Solutions, pH Range of Strong Acids, Role of pH in Our Digestive System and, Role of pH for Growth of Plants
Important Questions on Strength of Acids and Bases
What is full form of pH?
A student placed and in two seperate beakers as shown.
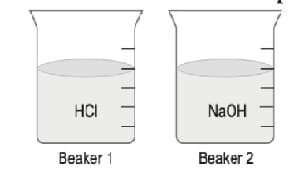
In beaker of NaOH is added whereas in beaker is added. The student notes the possible changes in in both solutions.
| S.No. |
Changes in pH (Beaker 1) |
Changes in pH (Beaker 2) |
| A | increase | increase |
| B | reduce | increase |
| C | increase | reduce |
| D | reduce | reduce |
Which change in pH is correct ?
What is hydrogen ion concentration of the solution when its is ?
The acid produced naturally in our stomach is:
Which acid enters into our body by a honey bee stung?
Two solutions A and B when tested with universal indicator shows their pH values are and respectively. What are A and B?
What happens when: Teeth are not cleaned after eating food at night.
What is pH? Describe the pH range of acidic and alkaline solutions.
In , what P and H indicates?
Which acid is found in the sting of a red ant?
is the negative logarithm of which ions?
Calculate the ion concentration of a solution having a pH of .
pH of blood varies between 7.2 to 7.4.
The acidic and basic strength of solution is based on _______________ ion concentration.
The first commercial pH meter was designed by Lavoisier.
pH is the negative logarithm of the _____ ion concentration.
What would you expect the pH of pure water to be? ()
Which acid is produced in our stomach?
